Tuberculosis (TB)
Tuberculosis is a chronic, progressive mycobacterial infection, often with an asymptomatic latent period following initial infection. Tuberculosis most commonly affects the lungs.
Etiology of TB:
Tuberculosis properly refers only to disease caused by
Mycobacterium tuberculosis (for which humans are the main reservoir). Similar disease occasionally results from the closely related mycobacteria,
M. bovis, M. africanum, and M. microti. These three bacteria, together with M. tuberculosis and other less common mycobacteria, are known as the Mycobacterium tuberculosis complex.
TB results almost exclusively from inhalation of airborne particles (
droplet nuclei) containing M. tuberculosis. They disperse primarily through coughing, singing, and other forced respiratory maneuvers by people who have active pulmonary or laryngeal TB and whose sputum contains a large number of organisms (about 10,000 organisms/mL, the limit of detection by fluorescent microscopy). People with pulmonary cavitary lesions are especially contagious because of the large number of bacteria contained within a lesion.
Droplet nuclei (particles < 5 micrometers in diameter) containing tubercle bacilli may remain suspended in room air currents for several hours, increasing the chance of spread. however, once these droplets land on a surface, it is difficult to resuspend the organisms (eg, by sweeping the floor, shaking out bed linens) as respirable particles. although such actions can resuspend dust particles containing tubercle bacilli, these particles are far too large to reach the alveolar surfaces necessary to initiate infection. contact with
fomites (eg, contaminated surfaces, food, personal respirators) do not appear to facilitate>
Untreated active pulmonary TB is highly variable in contagiousness. Certain strains of M. tuberculosis are more contagious, and patients with positive sputum smears are more contagious than those with positive results only on culture. Patients with cavitary disease (which is closely associated with mycobacterial burden in sputum) are more contagious than those without. Respiratory secretions with lower viscosity are more easily aerosolized, and the effectiveness of cough and other respiratory maneuvers in generating aerosol varies greatly.
Environmental factors also are important. Transmission is enhanced by frequent or prolonged exposure to untreated patients who are generating large numbers of tubercle bacilli in overcrowded, poorly ventilated, enclosed spaces; consequently, people living in poverty or in institutions are at particular risk. Health care practitioners who have close contact with active cases have increased risk.
Thus, estimates of contagiousness vary widely. Some studies suggest that only 1 in 3 patients with untreated pulmonary TB infect any close contacts, but the World Health Organization (WHO) estimates that each untreated patient may infect 10 to 15 people per year. However, most of those who are infected do not develop active disease.
Contagiousness decreases rapidly once effective treatment begins; cough decreases, and organisms are noninfectious even if they persist in sputum. Epidemiologic studies of household contacts suggest that transmission ends within 2 weeks of patients starting effective treatment, but more precise human-to-animal studies suggest that transmission ends within a few days of starting treatment.
Much less commonly, contagion results from aerosolization of organisms after irrigation of infected wounds, in mycobacteriology laboratories, or by aerosol or direct puncture in autopsy rooms.
TB of the tonsils, lymph nodes, abdominal organs, bones, and joints was once commonly caused by ingestion of milk or milk products (eg, cheese) contaminated with M. bovis, but this transmission route has been largely eradicated in countries where milk is pasteurized and cows that have a positive
tuberculin skin test result are slaughtered. Tuberculosis due to M. bovis still occurs in countries where bovine tuberculosis is endemic (eg, some Latin American countries) and in immigrants from those countries. The increasing popularity of cheese made from unpasteurized milk raises new concerns if the cheeses come from countries with a bovine TB problem (eg, Mexico, the United Kingdom). Bovine and human TB can be transmitted to other species such as badgers, deer, primates, and zoo animals. Slaughterhouses have been associated with zoonotic TB transmission.
Pathophysiology of TB:
Tuberculosis may occur in 3 stages:
•Primary infection
•Latent infection
•Active infection
M. tuberculosis bacilli initially cause a primary infection, a small percentage of which eventually progress to clinical disease of variable severity. However, most (about 95%) primary infections are asymptomatic. An unknown percentage of primary infections resolve spontaneously, but the majority are followed by a latent (dormant) phase. A variable percentage (5 to 10%) of latent infections subsequently reactivate with symptoms and signs of disease.
Infection is usually not transmissible in the primary stage and is never contagious in the latent stage.
Primary TB Infection:
Infection requires inhalation of particles small enough to traverse the upper respiratory defenses and deposit deep in the lungs, usually in the subpleural airspaces of the middle or lower lobes. Larger droplets tend to lodge in the more proximal airways and typically do not result in infection. Infection usually begins from a single droplet nucleus, which typically carries few organisms. Perhaps only a single organism may suffice to cause infection in susceptible people, but less susceptible people may require repeated exposure to develop infection.
To initiate infection, M. tuberculosis bacilli must be ingested by alveolar macrophages. Bacilli that are not killed by the macrophages actually replicate inside them, ultimately killing the host macrophage (with the help of CD8 lymphocytes); inflammatory cells are attracted to the area, causing a focal pneumonitis that coalesces into the characteristic tubercles seen histologically.
In the early weeks of infection, some infected macrophages migrate to regional lymph nodes (eg, hilar, mediastinal), where they access the bloodstream. Organisms may then spread hematogenously to any part of the body, particularly the apical-posterior portion of the lungs, epiphyses of the long bones, kidneys, vertebral bodies, and meninges. Hematogenous dissemination is less likely in patients with partial immunity due to vaccination or to prior natural infection with M. tuberculosis or environmental mycobacteria.
Latent infection:
occurs after most primary infections. In about 95% of cases, after about 3 weeks of uninhibited growth, the immune system suppresses bacillary replication, usually before symptoms or signs develop. Foci of bacilli in the lung or other sites resolve into epithelioid cell granulomas, which may have caseous and necrotic centers. Tubercle bacilli can survive in this material for years; the balance between the host’s resistance and microbial virulence determines whether the infection ultimately resolves without treatment, remains dormant, or becomes active. Infectious foci may leave fibronodular scars in the apices of one or both lungs (Simon foci, which usually result from hematogenous seeding from another site of infection) or small areas of consolidation (Ghon foci). A Ghon focus with lymph node involvement is a
Ghon complex, which, if calcified, is called a Ranke complex. The tuberculin skin test and interferon-gamma release blood assays (IGRA) become positive during the latent stage of infection. Sites of latent infection are dynamic processes and are not entirely dormant as was once believed.
Less often, the primary focus progresses immediately, causing acute illness with pneumonia (sometimes cavitary), pleural effusion, and marked mediastinal or hilar lymph node enlargement (which, in children, may compress bronchi). Small pleural effusions are predominantly lymphocytic, typically contain few organisms, and clear within a few weeks. This sequence may be more common among young children and recently infected or reinfected immunosuppressed patients.
Extrapulmonary TB at any site can sometimes manifest without evidence of lung involvement. TB lymphadenopathy is the most common extrapulmonary manifestation; however, meningitis is the most feared because of its high mortality in the very young and very old.
Active TB disease:
Healthy people who are infected with tuberculosis have about a 5 to 10% lifetime risk of developing active disease, although the percentage varies significantly by age and other risk factors.
In 50 to 80% of those who develop active disease, TB reactivates within the first 2 years, but it can also reactivate decades later.
Any organ initially seeded may become a site of reactivation, but reactivation occurs most often in the lung apices, presumably because of favorable local conditions such as high oxygen tension. Ghon foci and affected hilar lymph nodes are much less likely to be sites of reactivation.
Conditions that impair cellular immunity (which is essential for defense against TB) significantly facilitate reactivation. Thus, patients coinfected with HIV and not receiving appropriate antiretroviral therapy (ART) have about a 10% annual risk of developing active disease.
Other risk factors that facilitate reactivation, but to a lesser extent than HIV infection, include
Head and neck cancer
Gastrectomy
Jejunoileal bypass surgery
Dialysis-dependent chronic kidney disease
Significant weight loss
Use of drugs that suppress the immune system
Patients who require immunosuppression after solid organ transplantation are at the highest risk, but other immunosuppressants such as corticosteroids and tumor necrosis factor (TNF) inhibitors also commonly cause reactivation. Tobacco use also is a risk factor.
In some patients, active disease develops when they are reinfected rather than when latent disease reactivates. Reinfection is more likely to be the mechanism in areas where TB is prevalent and patients are exposed to a large inoculum of bacilli. Reactivation of latent infection predominates in low-prevalence areas. In a given patient, it is difficult to determine whether active disease resulted from reinfection or reactivation.
TB damages tissues through delayed-type hypersensitivity (DTH), typically producing granulomatous necrosis with a caseous histologic appearance. Lung lesions are characteristically but not invariably cavitary, especially in immunosuppressed patients with impaired DTH. Pleural effusion is less common than in progressive primary TB but may result from direct extension or hematogenous spread. Rupture of a large tuberculous lesion into the pleural space may cause empyema with or without bronchopleural fistula and sometimes causes pneumothorax. In the prechemotherapy era, TB empyema sometimes complicated medically induced pneumothorax therapy and was usually rapidly fatal, as was sudden massive hemoptysis due to erosion of a pulmonary artery by an enlarging cavity.
The course of TB varies greatly, depending on the virulence of the organism and the state of host defenses. The course may be rapid in members of isolated populations (eg, Native Americans) who, unlike many Europeans and their American descendents, have not experienced centuries of selective pressure to develop innate or natural immunity to the disease. The course is often more indolent in these European and American populations.
Acute respiratory distress syndrome (ARDS), which appears to be due to hypersensitivity to TB antigens, develops rarely after diffuse hematogenous spread or rupture of a large cavity with spillage into the lungs.
Symptoms and Signs of TB:
Primary infection is almost always asymptomatic, but when symptoms occur, they typically are nonspecific and include low-grade fever and fatigue without a prominent cough.
In active pulmonary tuberculosis, even moderate or severe disease, patients may have no symptoms, except “not feeling well,” along with anorexia, fatigue, and weight loss, which develop gradually over several weeks, or they may have more specific symptoms. Cough is most common. At first, it may be minimally productive of yellow or green sputum, usually when awakening in the morning, but cough may become more productive as the disease progresses. Hemoptysis occurs only with cavitary TB (due to granulomatous damage to vessels but sometimes due to fungal growth in a cavity).
Low-grade fever is common but not invariable. Drenching night sweats are a classic symptom but are neither common in nor specific for TB. Dyspnea may result from lung parenchymal damage, spontaneous pneumothorax, or pleural TB with effusion.
With HIV coinfection, the clinical presentation is often atypical because delayed hypersensitivity is impaired; patients are more likely to have symptoms of extrapulmonary or disseminated disease.
Extrapulmonary TB causes various systemic and localized manifestations depending on the affected organs.
Diagnosis:
Chest x-ray
Acid-fast stain and culture
Tuberculin skin test (TST) or interferon-gamma release assay (IGRA)
When available, nucleic acid amplification test (NAAT)
Pulmonary tuberculosis is often suspected based on one of the following:
Chest x-rays taken while evaluating respiratory symptoms (cough lasting > 3 weeks, hemoptysis, chest pain, dyspnea), an unexplained illness, fever of unknown origin (FUO), or a positive tuberculin skin test (TST)
IGRA done as a screening test or during contact investigation
Suspicion for TB is higher in patients who have fever, cough lasting > 2 to 3 weeks, night sweats, weight loss, and/or lymphadenopathy and in patients with possible TB exposure (eg, via infectious family members, friends, or other contacts; institutional exposure; or travel to TB-endemic areas).
Initial tests are chest x-ray and sputum examination and culture. If the diagnosis of active TB is still unclear after chest imaging and sputum examination, TST or IGRA may be done, but these are tests for infection not active disease. NAATs (eg, polymerase chain reaction [PCR]–based) are rapid and can be diagnostic.
Like most clinical tests, positive TB test results are statistically more likely to be false positives when the prior probability of TB infection is low (see also Understanding Medical Tests and Test Results).
Once TB is diagnosed, patients should be tested for HIV infection, and those with risk factors for hepatitis B or hepatitis C should be tested for those viruses. Baseline tests (eg, complete blood count, basic blood chemistry including hepatic and renal function) should be done.
Chest x-ray
In adults, a multinodular infiltrate above or behind the clavicle is most characteristic of active TB; it suggests reactivation of disease. It is best visualized in an apical-lordotic view or with chest CT.
Middle and lower lung infiltrates are nonspecific but should prompt suspicion of primary TB in patients (usually younger) whose symptoms or exposure history suggests recent infection, particularly if there is pleural effusion.
Calcified hilar nodes may be present; they may result from primary TB infection but may also result from histoplasmosis in areas where histoplasmosis is endemic (eg, the Ohio River Valley).
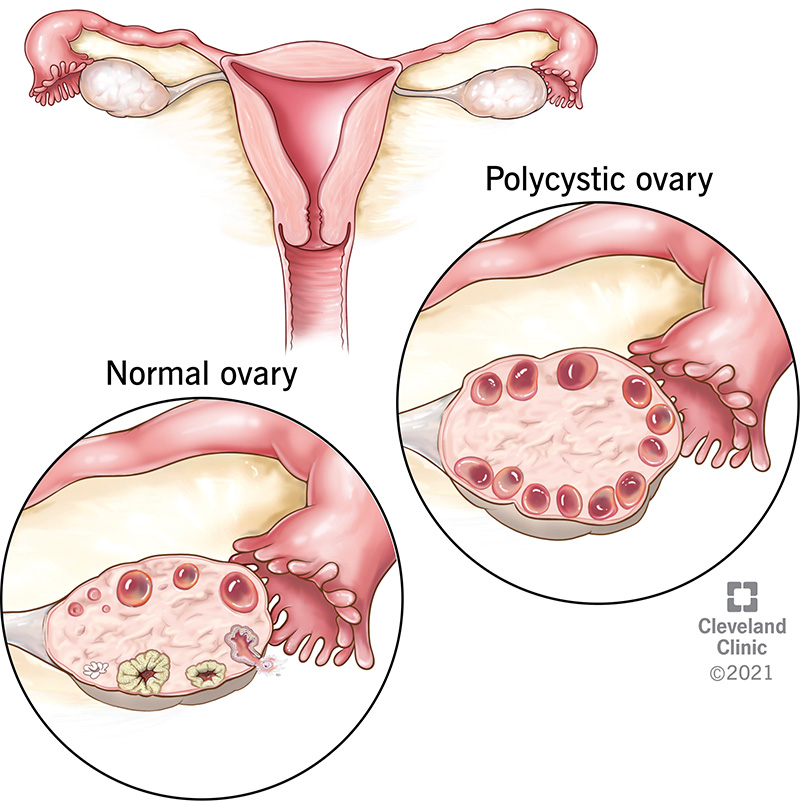
 |
A right upper lobe cavitary lesion on a chest x-ray of a patient with tuberculosis.
|
Sputum examination, culture, and testing
Sputum testing is the mainstay for diagnosis of pulmonary tuberculosis. A nonsputum-based diagnostic test has long been sought because sputum is often difficult to collect; breath and urine tests are available, and the urine tests have proved useful in diagnosing TB disease in people with HIV infection. If patients cannot produce sputum spontaneously, aerosolized hypertonic saline can be used to induce it. If induction is unsuccessful, bronchial washings, which are particularly sensitive, can be obtained by fiberoptic bronchoscopy. Because induction of sputum and bronchoscopy entail some risk of infection for medical staff, these procedures should be done as a last resort in selected cases. Appropriate precautions (eg, negative-pressure room, N-95 or other fitted respirators) should be used.
The first step in sputum examination is typically microscopic examination to check for acid-fast bacilli (AFB). Tubercle bacilli are nominally gram-positive but take up Gram stain inconsistently; samples are best prepared with Ziehl-Neelsen or Kinyoun stains for conventional light microscopy or fluorochrome stains for the more sensitive fluorescent microscopy. Smear microscopy can detect about 10,000 bacilli/mL of sputum, making it insensitive when fewer bacilli are present, as occurs in early reactivation or in patients with HIV coinfection.
Although finding AFB in a sputum smear is strong presumptive evidence of TB in the presence of TB risk factors, in other settings environmental mycobacteria may be more likely, and definitive diagnosis requires a positive mycobacterial culture or NAAT.
Culture is also required to isolate bacteria for conventional drug susceptibility testing and genotyping. However, molecular drug susceptibility testing is increasingly replacing culture-based methods. Culture can detect as few as 10 bacilli/mL of sputum and can be done using solid or liquid media. However, it can take up to 3 months for final confirmation of culture results. Liquid media are more sensitive and faster that solid media, with results available in 2 to 3 weeks. Rapid antigen testing to detect the MPB64 antigen can confirm that organisms growing on mycobacterial culture are M. tuberculosis.
Two types of NAAT are available for TB diagnosis:
Xpert MTB/RIF
Line probe assay
Skin testing:
Multiple-puncture devices (tine test) are no longer recommended.
The tuberculin skin test (TST—Mantoux intradermal method) using purified protein derivative (PPD) is usually done. The TST measures the immunologic response to M. tuberculosis and thus should be positive in both latent and active infection and so cannot distinguish between the two.
The standard dose in the US of 5 tuberculin units (TU) of PPD in 0.1 mL of solution is injected on the volar forearm. It is critical to give the injection intradermally, not subcutaneously. A well-demarcated bleb or wheal upon injection indicates a properly placed injection. The diameter of induration (not erythema) transverse to the long axis of the arm is measured 48 to 72 hours after injection. Use of a pen to demarcate the boundaries of induration on the skin can help produce more precise measurements, but reading skin tests is inherently variable and subject to a number of errors, including terminal digit preference, that is, a tendency to favor recording 5-, 10-, 15- and 20-mm results. In research studies, measurements done with a caliper or ruler where measurement numbers were not immediately visible to the reader produced less biased readings.
Given the difficulty of demarcating and accurately measuring the induration, it is ill-advised to attach clinical significance to minor differences. For example, a 9-mm reading should probably not be interpreted as different from an 11-mm reading (ie, treating the 11-mm reading as latent infection while dismissing the 9-mm reading as uninfected). Among household contacts, and in other settings where recent transmission is considered certain, TST results average about 17 mm in induration. Clinically, it is useful to remember that recently infected people are at greatest risk of reactivation and that, if they are immunocompetent, they usually have a vigorous immune response, manifested by a large TST or interferon gamma release test (IGRA) result.
TST responses tend to decrease over time, commonly long outlasting the presence of viable M. tuberculosis organisms capable of reactivation. Although late reactivation is well documented, most reactivation of latent infection occurs within a year to 18 months of initial infection. Treating latent TB many years after infection likely occurred may be advisable when immunosuppression is contemplated, but residual infection that is likely to reactivate may no longer be present. Reversion of skin tests that occurs in the absence of treatment or anergy (no reaction to any skin test) is often missed because follow-up testing is not done. Spontaneous cure is the likely reason. In settings of high transmission, disease often results from recent rather than remote infection, although both can occur.
Repeated administration of TSTs can cause the immune system to recall previous hypersensitivity that has waned over time, so-called boosting. Unrecognized, boosting can result in unnecessary treatment of contacts, for example, in the context of an outbreak investigation. To avoid misinterpreting boosting as recent infection in settings where serial testing is indicated, two-step baseline testing is recommended. The idea is to retest people with a negative TST result within a 1 to 4 weeks to see whether there is recall of previous hypersensitivity. If not, that result is a true negative. However, a positive TST result on retesting 1 to 4 weeks after the first test is assumed to indicate pre-existing latent infection and patients are treated or not treated based on clinical criteria. Boosting is not a problem with repeated IGRA testing because no antigens are injected.
Recommended cutoff points for a positive TST reaction depend on the clinical setting:
5 mm: Patients at high risk of developing active TB if infected, such as those who have chest x-ray evidence of past TB, who are immunosuppressed because of HIV infection or drugs (eg, TNF-alpha inhibitors, corticosteroid use equivalent to prednisone 15 mg/day for > 1 month), or who are close contacts of patients with infectious TB
10 mm: Patients with some risk factors, such as injection drug users, recent immigrants from high-prevalence areas, residents of high-risk settings (eg, prisons, homeless shelters), patients with certain disorders (eg, silicosis, renal insufficiency, diabetes, head or neck cancer), and those who have had gastrectomy or jejunoileal bypass surgery
15 mm: Patients with no risk factors (who typically should not be tested)
False-negative TST results can occur, most often in patients who are febrile, older, HIV-infected (especially if CD4 count is < 200 cells/mcL [0.2 x 109/L]), immune-suppressed because of a disorder or use of certain drugs (eg, corticosteroids, certain biologic immune modulators, certain cancer drugs), or very ill. Many of these people show no reaction to any skin test (anergy). Anergy probably occurs because inhibiting antibodies are present or because so many T cells have been mobilized to the disease site that too few remain to produce a significant skin reaction.
False-positive TST results may occur if patients have nontuberculous mycobacterial infections or have received the bacille Calmette-Guérin (BCG) vaccine. However, the effect of BCG vaccination on TST usually wanes after several years; after this time, a positive test is likely to be due to TB infection. Infection after BCG vaccination occurs in high-transmission settings. People arriving in the US from high-burden areas often cite BCG as the reason for their positive TST, rather than get stigmatized by a diagnosis of TB, and resist treatment for latent infection even when it is clearly indicated. Authorities suggest that BCG vaccination status should be ignored and infection assumed, but this leads to overdiagnosis of latent TB infection, unnecessary concern and treatment, and potential drug adverse effects. The use of IGRAs to diagnose TB infection, although not also without interpretation problems, has largely solved the BCG/latent TB infection controversy.
Treatment of TB:
First-line drugs for TB
The first-line drugs isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB) are used together in initial treatment. There are a several different TB treatment regimens, chosen based on numerous factors. Dosing of first-line drugs can be done at different intervals.
Isoniazid (INH) is given orally once/day, has good tissue penetration (including cerebrospinal fluid), and is highly bactericidal. It remains the single most useful and least expensive drug for TB treatment. Decades of uncontrolled use—often as monotherapy—in many countries (especially in East Asia) have greatly increased the percentage of resistant strains. In the US, about 10% of isolates are INH-resistant.
Adverse effects of isoniazid include rash, fever, and, rarely, anemia and agranulocytosis. INH causes asymptomatic, transient aminotransferase elevations in up to 20% of patients and clinical (usually reversible) hepatitis in about 1/1000. Clinical hepatitis occurs more often in patients > 35 years old, patients with alcohol use disorder, postpartum women, and patients with chronic liver disease. Monthly liver testing is not recommended unless patients have risk factors for liver disease. Patients with unexplained fatigue, anorexia, nausea, vomiting, or jaundice may have hepatic toxicity; treatment is suspended and liver tests are done. Those with symptoms and any significant aminotransferase elevation (or asymptomatic elevation > 5 times normal) by definition have hepatic toxicity, and INH is stopped.
After recovery from mild aminotransferase elevations and symptoms, patients can be safely challenged with a half dose for 2 to 3 days. If this dose is tolerated (typically in about half of patients), the full dose may be restarted with close monitoring for recurrence of symptoms and deterioration of liver function. If patients are receiving INH, RIF, and PZA, all drugs must be stopped, and the challenge done with each drug separately. INH or PZA, rather than RIF, is the more likely cause of hepatotoxicity.
Peripheral neuropathy can result from INH-induced pyridoxine (vitamin B6) deficiency, most likely in pregnant or breastfeeding women, undernourished patients, patients with diabetes mellitus or HIV infection, patients with alcohol use disorder, patients with cancer or uremia, and older patients. A daily dose of pyridoxine 25 to 50 mg can prevent this complication, although pyridoxine is usually not needed in children and healthy young adults.
INH delays hepatic metabolism of phenytoin, requiring dose reduction. It can also cause a violent reaction to disulfiram, a drug occasionally used for alcohol use disorder. INH is safe during pregnancy.
Rifampin (RIF), given orally, is bactericidal, is well-absorbed, penetrates well into cells and cerebrospinal fluid, and acts rapidly. It also eliminates dormant organisms in macrophages or caseous lesions that can cause late relapse. Thus, RIF should be used throughout the course of therapy.
Adverse effects of rifampin include cholestatic jaundice (rare), fever, thrombocytopenia, and renal failure. RIF has a lower rate of hepatotoxicity than INH. Drug interactions must be considered when using RIF. It accelerates metabolism of anticoagulants, oral contraceptives, corticosteroids, digitoxin, oral antihyperglycemic drugs, methadone, and many other drugs. The interactions of rifamycins and many antiretroviral drugs are particularly complex; combined use requires specialized expertise. RIF is safe during pregnancy.
The following newer rifamycins are available for special situations:
Rifabutin is used for patients taking drugs (particularly antiretroviral drugs) that have unacceptable interactions with RIF. Its action is similar to RIF, but it affects the metabolism of other drugs less. When used with clarithromycin or fluconazole, rifabutin has been associated with uveitis.
Rifapentine is used in one dose/week regimens and the new 4-month treatment regimen but is not used in children or patients with HIV (because of unacceptable treatment failure rates) or extrapulmonary TB. It is also used in a 12-dose, once/week DOT regimen with INH for TB prophylaxis. This prophylactic combination is not recommended for children < 2 years old, hiv-infected patients receiving antiretroviral treatment, pregnant women, or women expecting to become pregnant during treatment because safety in these groups is>
In 2020, nitrosamine impurities were found in samples of RIF and rifapentine. Some of these impurities have been implicated as possible carcinogens in long-term animal studies, with toxicity largely related to cumulative exposure. However, for treatment of TB disease, the Centers for Disease Control and Prevention (CDC) favors continued use of RIF, if acceptable to the patient, because exposure is time-limited and the risks of not taking RIF likely outweigh any potential risks of nitrosamine impurities.
not used in children or patients with HIV (because of unacceptable treatment failure rates) or extrapulmonary TB. It is also used in a 12-dose, once/week DOT regimen with INH for TB prophylaxis. This prophylactic combination is not recommended for children < 2 years old, hiv-infected patients receiving antiretroviral treatment, pregnant women, or women expecting to become pregnant during treatment because safety in these groups is>
In 2020, nitrosamine impurities were found in samples of RIF and rifapentine. Some of these impurities have been implicated as possible carcinogens in long-term animal studies, with toxicity largely related to cumulative exposure. However, for treatment of TB disease, the Centers for Disease Control and Prevention (CDC) favors continued use of RIF, if acceptable to the patient, because exposure is time-limited and the risks of not taking RIF likely outweigh any potential risks of nitrosamine impurities.
Pyrazinamide (PZA) is an oral bactericidal drug. When used during the intensive initial 2 months of treatment, it shortens the duration of therapy to 6 months and prevents development of resistance to RIF.
The major adverse effects of PZA are gastrointestinal upset and hepatitis. It often causes hyperuricemia, which is generally mild and only rarely induces gout. PZA is commonly used during pregnancy, but its safety has not been confirmed.
Ethambutol (EMB) is given orally and is the best-tolerated of the first-line drugs. Its main toxicity is optic neuritis, which is more common at higher doses (eg, 25 mg/kg) and in patients with impaired renal function. Patients with optic neuritis present initially with an inability to distinguish blue from green, followed by impairment of visual acuity. Because both symptoms are reversible if detected early, patients should have a baseline test of visual acuity and color vision and should be questioned monthly regarding their vision. Patients taking EMB for > 2 months or at doses higher than those listed in the table should have monthly visual acuity and color vision testing. Caution is warranted if communication is limited by language and cultural barriers. For similar reasons, EMB is usually avoided in young children who cannot read eye charts but can be used if needed because of drug resistance or drug intolerance. Another drug is substituted for EMB if optic neuritis occurs. Ethambutol can be used safely during pregnancy. Resistance to EMB is less common than to the other first-line drugs.
Second-line drugs for TB:
if needed because of drug resistance or drug intolerance. Another drug is substituted for EMB if optic neuritis occurs. Ethambutol can be used safely during pregnancy. Resistance to EMB is less common than to the other first-line drugs.
TABLE
Dosing of Oral First-Line Anti-TB Drugs*
Second-line drugs for TB
Other antibiotics are active against TB and are used primarily when patients have drug-resistant TB (DR-TB) or do not tolerate one of the first-line drugs. Until 2016, the 2 most important classes were the aminoglycosides and the closely related polypeptide drug, capreomycin (injectable only), and the fluoroquinolones.
Streptomycin, the first and once the most commonly used injectable, is now uncommonly used and is increasingly difficult to obtain because of its replacement by newer injectable and oral second-line drugs. It is very effective and bactericidal. Resistance is still relatively uncommon in the US but is more common globally. CSF penetration is poor, and intrathecal administration should not be used if other effective drugs are available.
Dose-related adverse effects of streptomycin include renal tubular damage, vestibular damage, and ototoxicity. The dose is about 15 mg/kg IM. The maximum is usually 1 g for adults, reduced to 0.75 g (10 mg/kg) for those ≥ 60 years. To limit dose-related adverse effects, clinicians give the drug only 5 days/week for up to 2 months. Then it may be given twice/week for another 2 months if necessary. In patients with renal insufficiency, dosing frequency should be reduced (eg, 12 to 15 mg/kg/dose 2 or 3 times/week). Patients should be monitored with appropriate testing of balance, hearing, and serum creatinine levels.
Adverse effects of streptomycin include rash, fever, agranulocytosis, and serum sickness. Flushing and tingling around the mouth commonly accompany injection but subside quickly. Streptomycin is contraindicated during pregnancy because it may cause vestibular toxicity and ototoxicity in the fetus.
Kanamycin and amikacin may remain effective even if streptomycin resistance has developed. Their renal and neural toxicities are similar to those of streptomycin. Kanamycin has been a widely used injectable for MDR-TB, but amikacin is rapidly replacing it in the increasingly uncommon situations in which injectables are needed.
Capreomycin, a related nonaminoglycoside parenteral bactericidal drug, has dosage, effectiveness, and adverse effects similar to those of aminoglycosides. It was an important drug for MDR-TB because isolates resistant to streptomycin are often susceptible to capreomycin, and it is somewhat better-tolerated than aminoglycosides when prolonged administration is required. Like all injectables, it is painful to administer and less well tolerated than the newer, oral, drug-resistant regimens, which are now typically preferred.
Some fluoroquinolones (levofloxacin, moxifloxacin) are the most active and safest TB drugs after isoniazid and rifampin; however, until the introduction of the new 4-month regimen containing moxifloxacin, fluoroquinolones were not first-line drugs for TB susceptible to isoniazid and rifampin. Moxifloxacin appears to be as active as isoniazid when used with rifampin or rifapentine.
Other second-line drugs include ethionamide, cycloserine, and para-aminosalicylic acid (PAS). These drugs are less effective and more toxic than other anti-TB drugs but were essential until the advent of all-oral regimens (see below).
Newer anti-TB drugs include bedaquiline, delamanid, pretomanid, and sutezolid. These had been reserved for highly resistant TB or for patients who cannot tolerate other second-line drugs but are increasingly being used in the all-oral drug-resistant regimens.
Drug resistance:
ver, in any given patient, the most common reason for drug-resistant TB (DR-TB) is acquisition by person-to-person transmission, often from unsuspected, undiagnosed, or inadequately treated people with DR-TB. Globally, only one third of people with MDR-TB have access to effective treatment. In areas where resistance testing is inadequate or unavailable, many patients who do not respond to first-line therapy probably have unrecognized MDR-TB and are contagious to others, including reinfection of people with drug-susceptible TB. The use of rapid molecular testing for TB and rifampin resistance has been shown to reduce the propagation of DR-TB.
Drug-resistance categories are defined based on the antibiotics to which an organism is resistant. In January 2021, the WHO revised its definition of XDR-TB and formally defined pre-XDR-TB (4). In the US in January 2022, the CDC recommended hybrid definitions, allowing use of the WHO definitions or alternatives appropriate in the US:
Multidrug-resistant TB (MDR-TB): Resistance to isoniazid and rifampin, the two most effective first-line drugs, with or without resistance to other drugs
Pre-XDR-TB: Resistance to isoniazid and rifampin and also any fluoroquinolone; CDC alternative definition: Resistance to isoniazid, rifampin, and a second-line injectable (amikacin, capreomycin, and kanamycin)
Extensively drug-resistant tuberculosis (XDR-TB): Resistance to isoniazid, rifampicin, any fluoroquinolone, and at least one additional group A drug (The group A drugs are levofloxacin, moxifloxacin, bedaquiline, and linezolid. They are the most potent of the second-line drugs used for drug-resistant TB and require longer treatment regimens.); CDC alternative definition: Resistance to isoniazid, rifampin, any fluoroquinolone, and either bedaquiline or linezolid (or both)
The diagnosis of MDR-TB and consequent need to use second-line drugs has great significance in terms of length, cost, and success of treatment. However, the new, shorter, all-oral DR-TB regimens have made treatment less difficult and rendered those issues less of a dividing line between clinical success and failure.
Treatment regimens:
Until the introduction of the new, 4-month regimen, treatment of all patients with new, previously untreated TB had consisted of the following:
2-month initial intensive phase
4- to 7-month continuation phase
Initial intensive–phase therapy is with 4 antibiotics (see table for dosing):
Isoniazid (INH)
Rifampin (RIF)
Pyrazinamide (PZA)
Ethambutol (EMB)
growth (bacterial growth is often delayed well after antibiotics are below the minimal inhibitory concentration). However, daily therapy is recommended for patients with MDR-TB or HIV coinfection. Regimens involving less than daily dosing must be carried out as directly observed therapy (DOT) because each dose becomes more important.
After 2 months of intensive 4-drug treatment, PZA and usually EMB are stopped, depending on the drug susceptibility pattern of the original isolate.
Continuation-phase treatment depends on
Results of drug susceptibility testing of initial isolates (where available)
The presence or absence of a cavitary lesion on the initial chest x-ray
Results of cultures and smears taken at 2 months
If positive, 2-month cultures indicate the need for a longer course of treatment.
If both culture and smear are negative, regardless of the chest x-ray, or if the culture or smear is positive but x-ray showed no cavitation, INH and RIF are continued for 4 more months (6 months total).
If the x-ray showed cavitation and the culture or smear is positive, INH and RIF are continued for 7 more months (9 months total).
In either regimen, EMB is usually stopped if the initial culture shows no resistance to any drug. Continuation-phase drugs can be given daily or, if patients are not HIV-positive, 2 or 3 times/week. Patients who have negative culture and smears at 2 months and no cavitation on chest x-ray and who are HIV-negative may receive INH plus rifapentine once/week.
Patients who have positive cultures after 2 months of treatment should be evaluated to determine the cause. Evaluation for MDR-TB, a common cause, should be thorough. Clinicians should also check for other common causes (eg, nonadherence, extensive cavitary disease, drug resistance, malabsorption of drugs).
For both initial and continuation phases, the total number of doses (calculated by doses/week times number of weeks) should be given; thus if any doses are missed, treatment is extended and not stopped at the end of the time period.